Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 00:00, PineappleDevil889
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
Do you know the correct answer?
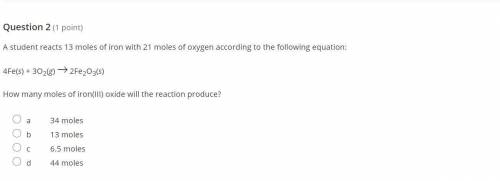
A student reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
4Fe(...
Questions in other subjects:




Mathematics, 16.03.2020 18:50






Mathematics, 16.03.2020 18:50