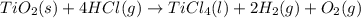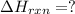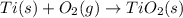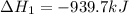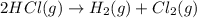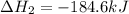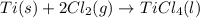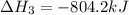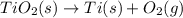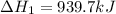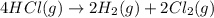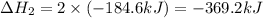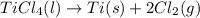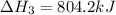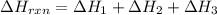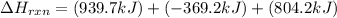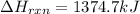Chemistry, 16.03.2020 18:50, kyleighott
Determine the heat of reaction for the process TiO2(s) + 4HCl(g) TiCl4(l) + 2H2(g) + O2(g) using the information given below: Ti(s) + O2(g) TiO2(s) H° = −939.7 kJ 2HCl(g) H2(g) + Cl2(g) H° = −184.6 kJ Ti(s) + 2Cl2(g) TiCl4(l) H° = −804.2 kJ

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2


Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
Do you know the correct answer?
Determine the heat of reaction for the process TiO2(s) + 4HCl(g) TiCl4(l) + 2H2(g) + O2(g) using the...
Questions in other subjects:


Mathematics, 02.05.2021 14:00

Mathematics, 02.05.2021 14:00

Mathematics, 02.05.2021 14:00

History, 02.05.2021 14:00



Physics, 02.05.2021 14:00

History, 02.05.2021 14:00

English, 02.05.2021 14:00