Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
Do you know the correct answer?
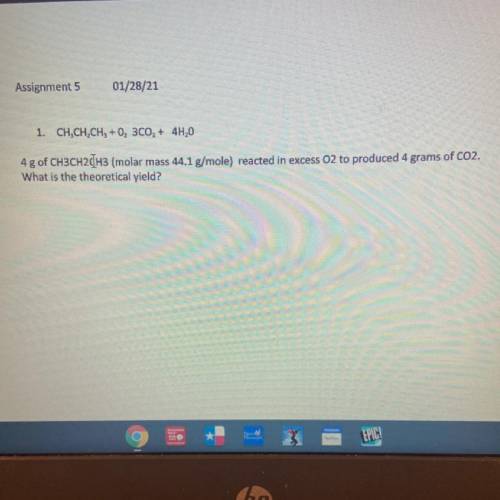
1. CH, CH, CH3 + 0, 300, + 4H,0
4g of CH3CH2CH3 (molar mass 44.1 g/mole) reacted in excess O2 to pr...
Questions in other subjects:

Mathematics, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00


Mathematics, 18.02.2021 14:00



Mathematics, 18.02.2021 14:00

Physics, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00