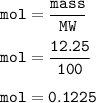Chemistry, 15.12.2020 14:00, royaltyy6533
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heated
and forms calcium oxide, Cao, and carbon dioxide, CO2.
a. Write a balanced chemical equation to demonstrate this reaction. Include state symbols.
b. Calculate the number of moles of calcium carbonate that is thermally decomposed in this
reaction.
Ok

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
Do you know the correct answer?
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heate...
Questions in other subjects:

Mathematics, 17.09.2020 14:01

History, 17.09.2020 14:01

Physics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01