
Chemistry, 06.12.2020 14:00, cthompson1107
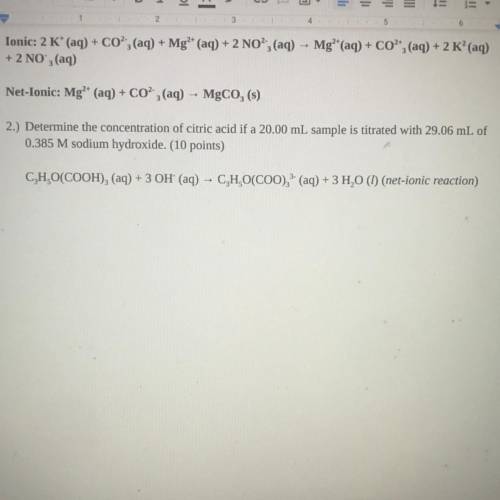
2.) Determine the concentration of citric acid if a 20.00 mL sample is titrated with 29.06 mL of
0.385 M sodium hydroxide. (10 points)
C, H,O(COOH)2 (aq) + 3 OH' (aq) - C, H,O(COO), (aq) + 3 H20 (1) (net-ionic reaction)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2
Do you know the correct answer?
2.) Determine the concentration of citric acid if a 20.00 mL sample is titrated with 29.06 mL of
0....
Questions in other subjects:

Chemistry, 17.03.2022 14:00

Social Studies, 17.03.2022 14:00

Geography, 17.03.2022 14:00


Mathematics, 17.03.2022 14:00



Arts, 17.03.2022 14:00







