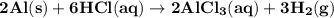Chemistry, 26.11.2020 08:00, jladinosolarsee
Help pls!! Due in 30 mins!
Which equation represents a single replacement reaction?
1. 2Al(s) + 3Cl2(g) → 2AlCl3(s)
2. 2Al(s) + 6HCl(aq) + 2AlCl3(aq) + 3H2(g)
3. 2AlCl3(s) → 2Al(s) + Cl2(g)
4. AlCl3(aq) + 3KOH(aq) + Al(OH)3(s) + 3KCl(aq)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 03:30, acaciacoats
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2
Do you know the correct answer?
Help pls!! Due in 30 mins!
Which equation represents a single replacement reaction?
1. 2Al(s)...
1. 2Al(s)...
Questions in other subjects:



Mathematics, 06.09.2020 20:01

Mathematics, 06.09.2020 20:01


Mathematics, 06.09.2020 20:01

Social Studies, 06.09.2020 20:01


Mathematics, 06.09.2020 20:01