
Chemistry, 18.11.2020 17:10, Leggett3146
Be sure to answer all parts. The equilibrium constant Kc for the reaction below is 61.5 at a certain temperature. H2(g) + I2(g) ⇆ 2HI(g) If you start with 0.155 M hydrogen iodide, what will the concentrations of H2, I2, and HI be at equilibrium?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2


Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
Be sure to answer all parts. The equilibrium constant Kc for the reaction below is 61.5 at a certain...
Questions in other subjects:

Mathematics, 14.07.2019 18:30




History, 14.07.2019 18:30

Biology, 14.07.2019 18:30



Physics, 14.07.2019 18:30


![[I_2]=[H_2]=0.0157M](/tpl/images/0908/7306/da87a.png)
![[HI]=0.124M](/tpl/images/0908/7306/87ea7.png)
![Kc=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0908/7306/bf8a4.png)
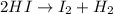
![\frac{1}{Kc}=0.0163 =\frac{[I_2][H_2]}{[HI]^2}](/tpl/images/0908/7306/4707b.png)
![[I_2]=[H_2]=x](/tpl/images/0908/7306/e08d5.png)
![[HI]=0.155-2x](/tpl/images/0908/7306/ef79b.png)
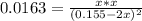
 :
: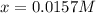
![[HI]=0.155-2(0.0157)=0.124M](/tpl/images/0908/7306/cba75.png)




