
Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2

Chemistry, 23.06.2019 07:30, Ayyyyeeeeeeewuzgud
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1

Chemistry, 23.06.2019 14:00, emory238
How are casts formed by decaying organisms? organisms turn into rock over time. organisms leave carbon residue on a rock. organisms leave impressions in sediment that hardens into rock. impressions left by organisms are filled in with sediment that hardens into rock.
Answers: 2
Do you know the correct answer?
Calculate the mass of 1.35 moles of sodium chloride...
Questions in other subjects:

Mathematics, 21.08.2020 22:01



Social Studies, 21.08.2020 22:01



Health, 21.08.2020 22:01

Mathematics, 21.08.2020 22:01

Mathematics, 21.08.2020 22:01


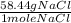 = 78.894 g of NaCl
= 78.894 g of NaCl





