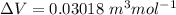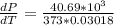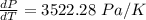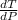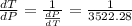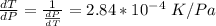Chemistry, 26.10.2020 16:40, freddyfriendo2364
10. What is the change in the boiling point of water at 1000 C per Pa change under atmospheric pressure conditions? The molar enthalpy of vaporization is 40.69 kJ mol-1, the molar volume of liquid water is 0.019 x 10-3 m3 mol-1, and the molar volume of steam is 30.199 x 10-3m3 mol-1, all at 1000 C and 1.01325 bar. (Hint: change in temperature per change in pressure)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, emmamerida
If 0.414 g of hydrogen is obtained in this experiment how many grams of sulfur must be obtained
Answers: 2

Chemistry, 21.06.2019 18:30, markmlg122
Two things that biomedical has invented or innvated
Answers: 1

Do you know the correct answer?
10. What is the change in the boiling point of water at 1000 C per Pa change under atmospheric press...
Questions in other subjects:


Social Studies, 16.02.2021 21:50

History, 16.02.2021 21:50



Mathematics, 16.02.2021 21:50




English, 16.02.2021 21:50


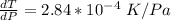

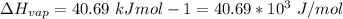
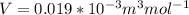
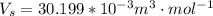
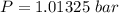
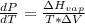
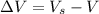
![\Delta V = [30.199 * 10^{-3} ] - [ 0.019 * 10^{-3}]](/tpl/images/0840/1908/33c9b.png)