
Chemistry, 18.10.2020 14:01, questions61
Explain how your observations verify that the residue in your crucible after heating is potassium chloride.
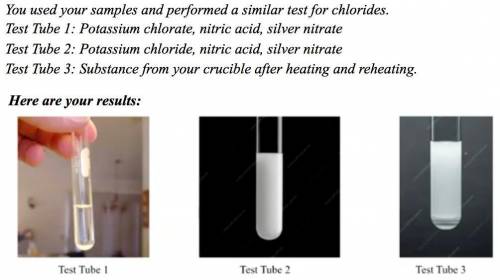

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1


Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
Do you know the correct answer?
Explain how your observations verify that the residue in your crucible after heating is potassium ch...
Questions in other subjects:



Geography, 25.03.2020 01:35

English, 25.03.2020 01:35




Spanish, 25.03.2020 01:35








