
Chemistry, 25.03.2020 01:35, naiomireyes74p2aybs
An element has the following successive ionization energies: I1 = 578 kJ/mol I2 = 1817 kJ/mol I3 = 2745 kJ/mol I4 = 11,577 kJ/mol I5 = 14,125 kJ/mol Based on these relative values, which ionization energies correspond to removing core (nonvalence) electrons?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3


Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
Do you know the correct answer?
An element has the following successive ionization energies: I1 = 578 kJ/mol I2 = 1817 kJ/mol I3 = 2...
Questions in other subjects:






Mathematics, 23.08.2019 22:00


Health, 23.08.2019 22:00

English, 23.08.2019 22:00


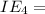 11,577 kJ/mol
11,577 kJ/mol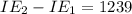 kJ/mol
kJ/mol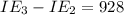 kJ/mol
kJ/mol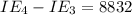 kJ/mol
kJ/mol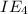 indicates the removal of core electron.
indicates the removal of core electron.



