
Chemistry, 17.10.2020 15:01, Packergood
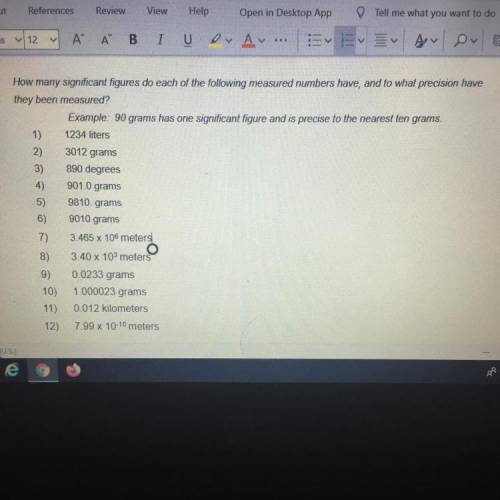
How many significant figures do each of the following measured numbers have, and to what precision have
they been measured?
Example: 90 grams has one significant figure and is precise to the nearest ten grams.
1) 1234 liters
2)
3)
4)
3012 grams
890 degrees
901.0 grams
9810. grams
9010 grams
5)
6)
7)
3.465 x 106 meters
8)
3.40 x 103 meters
9)
10)
0.0233 grams
1.000023 grams
0.012 kilometers
7.99 x 10-10 meters
11)
12)
U. S.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
Do you know the correct answer?
How many significant figures do each of the following measured numbers have, and to what precision h...
Questions in other subjects:









Mathematics, 22.01.2020 22:31






