
Chemistry, 16.10.2020 18:01, Clover1072
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tablet. The chlorate is titrated with iodide ions until the end point. The student reported that 29.50 mL of 0.100 M KI solution was required to reach the end point of a titration when 10 allergy tablets containing chlorate as the main active ingredient are dissolved in 25.00 mL of distilled water. What mass in grams of chlorate is present in the 10 allergy tablets?
Use the Balanced chemical equation to answer the question.
6, 1, 6, 3, 1, 3
Hint: this is a net ionic equation, so spectator ions have been removed!
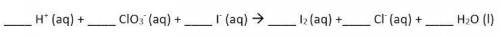

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Do you know the correct answer?
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tabl...
Questions in other subjects:

History, 28.12.2019 09:31


English, 28.12.2019 09:31


Biology, 28.12.2019 09:31





Biology, 28.12.2019 09:31






