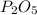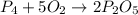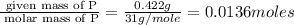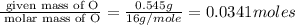Chemistry, 29.08.2019 07:50, jessezarate4513
When 0.422g of phosphorus is burned, 0.967g of a white oxide is obtained.
a. determine the empirical formula of the oxide
b. write a balanced equation for the reaction of phosphorus and molecular oxygen on the basis of this empirical formula

Answers: 1
Similar questions



Do you know the correct answer?
When 0.422g of phosphorus is burned, 0.967g of a white oxide is obtained.
a. determine the em...
a. determine the em...
Questions in other subjects:

English, 05.12.2020 03:20

Mathematics, 05.12.2020 03:20

Geography, 05.12.2020 03:20


SAT, 05.12.2020 03:20


Mathematics, 05.12.2020 03:20