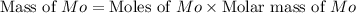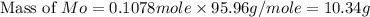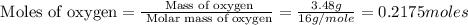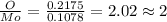Chemistry, 19.09.2019 03:30, Kekkdkskdkdk
A12.95 g sample of mo2o3(s) is converted completely to another molybdenum oxide by adding oxygen. the new oxide has a mass of 13.82 g. add subscripts below to correctly identify the empirical formula of the new oxide.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
Do you know the correct answer?
A12.95 g sample of mo2o3(s) is converted completely to another molybdenum oxide by adding oxygen. th...
Questions in other subjects:

English, 05.06.2020 23:03








Mathematics, 05.06.2020 23:03


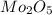 .
.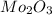 .
.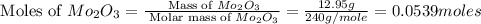
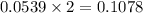 mole 'Mo'
mole 'Mo'