
Chemistry, 02.10.2020 14:01, haleyehewitt2001
Attached photo - Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell potential for a voltaic cell made from these two systems.
A. –1.68 V
B. –0.78 V
C. 0.78 V
D. 1.68 V
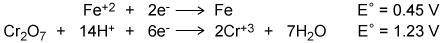

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
Do you know the correct answer?
Attached photo - Using the two cell reduction potentials shown for their corresponding reaction, cal...
Questions in other subjects:


Mathematics, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00

Social Studies, 09.03.2021 19:00



Mathematics, 09.03.2021 19:00


Mathematics, 09.03.2021 19:00







