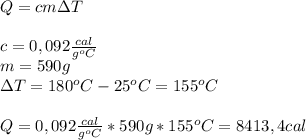Chemistry, 17.10.2019 17:50, 21hendlill
The specific heat of copper is 0.092 cal/g °c. if a 590 g copper pan is heated from a temperature of 25 °c to 180 °c, how many calories of heat energy will the copper pan absorb?

Answers: 1
Similar questions



Do you know the correct answer?
The specific heat of copper is 0.092 cal/g °c. if a 590 g copper pan is heated from a temperature of...
Questions in other subjects:

Mathematics, 24.04.2020 17:58


English, 24.04.2020 17:59

Mathematics, 24.04.2020 17:59






Chemistry, 24.04.2020 17:59