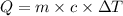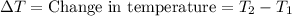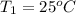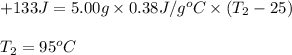Chemistry, 19.07.2019 01:20, katelynn73
A5.00-g sample of copper metal at 25.0 °c is heated by the addition of 133 j of energy. the final temperature of the copper is °c. the specific heat capacity of copper is 0.38 j/g°c.

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
Do you know the correct answer?
A5.00-g sample of copper metal at 25.0 °c is heated by the addition of 133 j of energy. the final te...
Questions in other subjects:



Mathematics, 05.01.2021 02:20

Mathematics, 05.01.2021 02:20

English, 05.01.2021 02:20

Mathematics, 05.01.2021 02:20

Mathematics, 05.01.2021 02:20

Mathematics, 05.01.2021 02:20


History, 05.01.2021 02:20