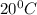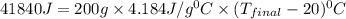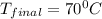Chemistry, 03.07.2020 22:01, Nashae4771
If 200. g of water at 20°C absorbs 41 840 J of energy, what will its final temperature be? (Specific Heat of water is 4.184 J/g*C)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
Do you know the correct answer?
If 200. g of water at 20°C absorbs 41 840 J of energy, what will its final temperature be? (Specific...
Questions in other subjects:











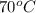
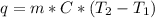
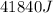
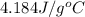
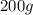
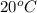
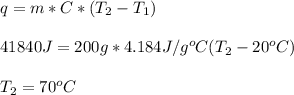
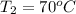

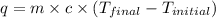
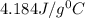
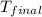 = final temperature =?
= final temperature =?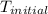 = initial temperature =
= initial temperature =