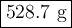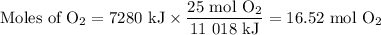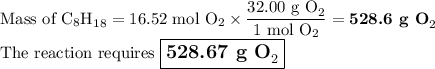Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
Do you know the correct answer?
A sample of octane undergoes combustion according to the equation 2 C8H18 + 25 O2 → 16 CO2 + 18 H2O...
Questions in other subjects:


Computers and Technology, 25.03.2020 23:58



Biology, 25.03.2020 23:58


Advanced Placement (AP), 25.03.2020 23:58