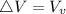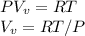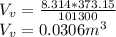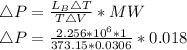Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling point of water by 1° C? You may assume that these changes are both small, so you only need to compute first derivatives. Remember that, under most conditions, the volume (per molecule) of liquid water is small compared to that of water vapor. Remember: Atmospheric pressure = 101300 Pa Boiling point at atmospheric pressure = 373.15 K Latent heat (LB) of boiling water = 2.256 × 106 J/kg Molecular weight (mw) of water = 0.018 kg/mol

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1


Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
Do you know the correct answer?
Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling...
Questions in other subjects:

Social Studies, 26.02.2020 01:30


Mathematics, 26.02.2020 01:30




Mathematics, 26.02.2020 01:30




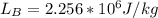
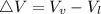
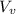 ) is far greater than the volume of liquid (
) is far greater than the volume of liquid (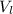 )
)