Order the molecules from the strongest intermolecular force (top) to the weakest (bottom).
Refe...

Chemistry, 03.05.2020 13:03, seanholmes91405
Order the molecules from the strongest intermolecular force (top) to the weakest (bottom).
Refer the table of electronegativities to help you.
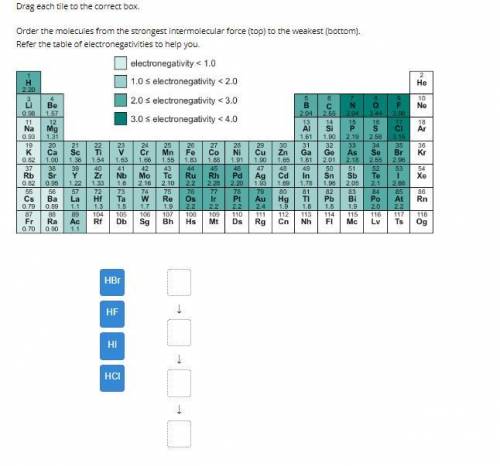

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
Do you know the correct answer?
Questions in other subjects:


History, 18.10.2019 06:00






Computers and Technology, 18.10.2019 06:10


Computers and Technology, 18.10.2019 06:10






