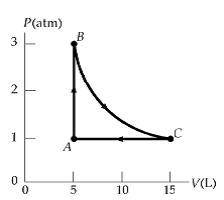
II. Practice An ideal gas occupies 5 L at atmospheric pressure and 300 K (point A). It is warmed at constant volume to 3 atm (point B). Then it is allowed to expand isothermally to 1 atm (point C) and at last compressed isobarically to its original state. A. How many moles of gas are being used? B. Find the temperature at point C. C. Find the work done on the gas in each process. D. Find the amount of heat added to/removed from the gas in one cycle.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


Chemistry, 23.06.2019 08:00, ira51
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2

Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
Do you know the correct answer?
II. Practice An ideal gas occupies 5 L at atmospheric pressure and 300 K (point A). It is warmed at...
Questions in other subjects:

Mathematics, 03.01.2020 01:31


Social Studies, 03.01.2020 01:31

Biology, 03.01.2020 01:31


Medicine, 03.01.2020 01:31



English, 03.01.2020 01:31