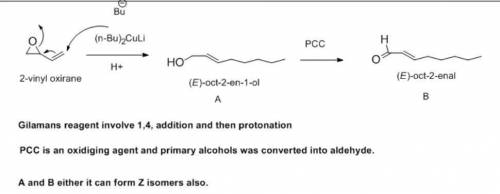
When the epoxide 2-vinyloxirane reacts with lithium dibutylcuprate, followed by protonolysis, a compound A is the major product formed. Oxidation of A with PCC yields B, a compound that gives a positive Tollens test and has an intense UV absorption around 215 nm. Treatment of B with Ag2O, followed by catalytic hydrogenation, gives octanoic acid. Identify A and B.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2
Do you know the correct answer?
When the epoxide 2-vinyloxirane reacts with lithium dibutylcuprate, followed by protonolysis, a comp...
Questions in other subjects: