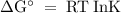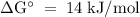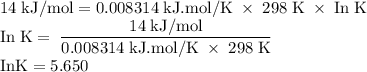Chemistry, 13.04.2020 22:28, ksoodagoat
The standard biological reaction Gibbs energy for the removal of the phosphate group from adenosine monophosphate is 14 kJ mol-1 at 298 K. What is the value of the thermodynamic standard reaction Gibbs energy?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
Do you know the correct answer?
The standard biological reaction Gibbs energy for the removal of the phosphate group from adenosine...
Questions in other subjects:











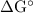 ) has been given by:
) has been given by: