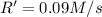Chemistry, 11.11.2019 12:31, KKHeffner02
The reaction a→b has been experimentally determined to be second order. the initial rate is 0.0100m/s at an initial concentration of a of 0.300m. determine the initial rate at [a]=0.900m.

Answers: 2
Similar questions




Chemistry, 10.10.2019 05:00, bcampos5397
Answers: 2
Do you know the correct answer?
The reaction a→b has been experimentally determined to be second order. the initial rate is 0.0100m/...
Questions in other subjects:







Mathematics, 09.07.2019 21:30


Mathematics, 09.07.2019 21:30

Mathematics, 09.07.2019 21:30


![R=k[A]^2](/tpl/images/0368/9972/0df80.png)
![0.0100M/s=k[0.300 M]^2](/tpl/images/0368/9972/1aa4f.png)
![k=\frac{0.0100M/s}{[0.300 M]^2}=0.1111 M^{-1} s^{-1}](/tpl/images/0368/9972/74690.png)
![R'=k[A]^2](/tpl/images/0368/9972/c61eb.png)
![R'=0.1111 M^{-1} s^{-1}\times [0.900 M]^2](/tpl/images/0368/9972/137eb.png)