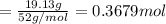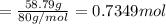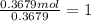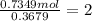Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2


Chemistry, 23.06.2019 06:20, ratpizza
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 07:00, MathChic68
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
Do you know the correct answer?
A 19.13 gram sample of chromium is heated in the presence of excess bromine. A metal bromide is form...
Questions in other subjects:


Social Studies, 26.10.2020 17:00



Advanced Placement (AP), 26.10.2020 17:00

Mathematics, 26.10.2020 17:00

Mathematics, 26.10.2020 17:00


English, 26.10.2020 17:00


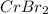 .
.