Ok another chemistry question! This is another question that I can)t solve
...

Chemistry, 31.03.2020 20:20, stefan19367
Ok another chemistry question! This is another question that I can)t solve
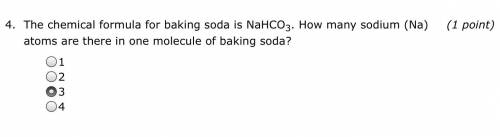

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

History, 08.10.2019 07:30


Chemistry, 08.10.2019 07:30


History, 08.10.2019 07:30

Social Studies, 08.10.2019 07:30


English, 08.10.2019 07:30


Biology, 08.10.2019 07:30






