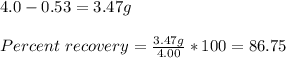Chemistry, 30.12.2019 17:31, SunsetPrincess
The solubility of acetanilide in hot water (5.5 g/100 ml at 100 c) is not very great, and it has some solubility in cold water (0.53 g/100 ml at 0 c). what would be the maximum theoretical percent recovery from the crystallization of 4.0 g of acetanilide from 100 ml of water (assuming the solution is chilled to 0 c for filtration). show your calculations.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
Do you know the correct answer?
The solubility of acetanilide in hot water (5.5 g/100 ml at 100 c) is not very great, and it has som...
Questions in other subjects:

Chemistry, 05.05.2021 20:00

Mathematics, 05.05.2021 20:00

Social Studies, 05.05.2021 20:00