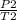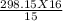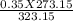Gay-Lussac Problems
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you in °C to convert
1. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0
°C to 30.0 °C.
2. If a gas in a closed container is pressurized from 15.0 atmospheres to 16.0 atmospheres and its
original temperature was 25.0 °C, what would the final temperature of the gas be?
3. A gas has a pressure of 0.35 atm at 50.0 °C. What is the pressure at standard temperature?
Boyle's law Drobleme

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
Do you know the correct answer?
Gay-Lussac Problems
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you...
P./T1 = P2/T2 - Remember, T is in KELVIN, add 273 to any temp given to you...
Questions in other subjects:

Mathematics, 16.10.2020 05:01


Biology, 16.10.2020 05:01







History, 16.10.2020 05:01


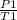 =
=