Chemistry, 26.03.2020 23:42, nadarius2017
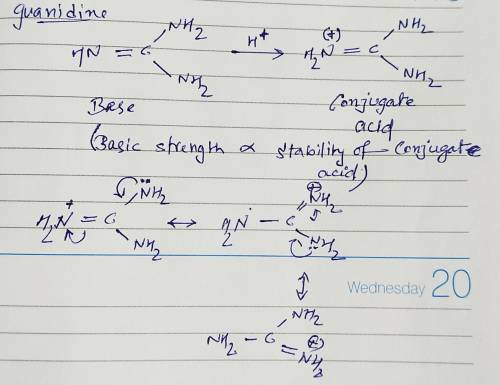
Guanidine is a stronger base than the typical amine. The increased basicity can be explained by drawing the resonance structures of the protonated guanidine. The protonated guanidine (A) has been drawn for you. Draw major resonance structures, one each in boxes B and C, and one minor resonance structure in Box D. Be sure to include the formal charge, lone pairs, and hydrogens on nitrogen for structures B, C, and D.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
Do you know the correct answer?
Guanidine is a stronger base than the typical amine. The increased basicity can be explained by draw...
Questions in other subjects:

Mathematics, 20.07.2019 18:00


Mathematics, 20.07.2019 18:00

Mathematics, 20.07.2019 18:00


History, 20.07.2019 18:00

Health, 20.07.2019 18:00


Mathematics, 20.07.2019 18:00