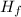Chemistry, 19.03.2020 05:38, Marshmallow9174
Given that the heat of fusion of water is +6.02 kJ/mol that the heat capacity of H2O(l)H2O(l) is 75.2 J/mol⋅KJ/mol⋅K and that the heat capacity of H2O(s)H2O(s) is 37.7 J/mol⋅KJ/mol⋅K, calculate the heat of fusion of water at -15 ∘C∘C.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
Do you know the correct answer?
Given that the heat of fusion of water is +6.02 kJ/mol that the heat capacity of H2O(l)H2O(l) is 75....
Questions in other subjects:






History, 02.03.2020 17:00


History, 02.03.2020 17:00



 ·(T₂ - T₁)
·(T₂ - T₁)