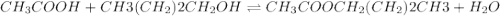Which one of the following choices describes most accurately the actual, internal reaction temperature (in other words, the temperature of the reaction mixture inside the reaction vial) for the Fischer esterification experiment of 1-butanol with acetic acid to form n-butyl acetate? Select one, and explain your answer.
a) Sand bath temperature (160-180 °C)
b) Boiling point of 1-butanol (116-118 °C)
c) Boiling point of the reaction mixture (reflux temperature)
d) Boiling point of acetic acid (117 °C)
e) Boiling point of n-butyl acetate (124-126 °C)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1


Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 06:00, womankrush538
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
Do you know the correct answer?
Which one of the following choices describes most accurately the actual, internal reaction temperatu...
Questions in other subjects:









Engineering, 14.09.2019 03:10