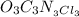Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1
Do you know the correct answer?
The empirical formula for trichloroisocyanuric acid, the active ingredient in many household bleache...
Questions in other subjects:




History, 07.07.2019 09:00



History, 07.07.2019 09:00

Physics, 07.07.2019 09:00

Biology, 07.07.2019 09:00

Mathematics, 07.07.2019 09:00


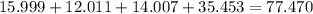
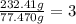
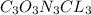 is the empirical formula.
is the empirical formula.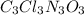
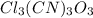
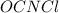 :
: