
Chemistry, 12.03.2020 17:28, kailinaguilar2187
For the following reaction, 5.62 grams of oxygen gas are mixed with excess ammonia. The reaction yields 3.58 grams of nitrogen monoxide. ammonia (g) oxygen (g) nitrogen monoxide (g) water (g) What is the ideal yield of nitrogen monoxide

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1


Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 03:00, rayne40
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
Do you know the correct answer?
For the following reaction, 5.62 grams of oxygen gas are mixed with excess ammonia. The reaction yie...
Questions in other subjects:

Biology, 29.03.2021 02:20



Computers and Technology, 29.03.2021 02:20

Chemistry, 29.03.2021 02:20


Mathematics, 29.03.2021 02:20

Mathematics, 29.03.2021 02:20


Biology, 29.03.2021 02:20


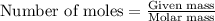 .....(1)
.....(1)

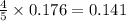 moles of nitrogen monoxide
moles of nitrogen monoxide




