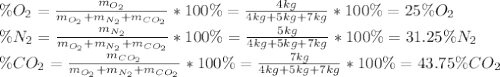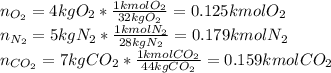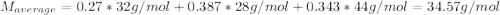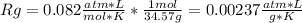Chemistry, 12.03.2020 00:29, damiangibson2
A gas mixture consists of 4 kg of O2, 5 kg of N2, and 7 kg of CO2. Determine (a) the mass fraction of each component, (b) the mole fraction of each component, and (c) the average molar mass and gas constant of the mixture.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 09:30, tramqpham25
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2
Do you know the correct answer?
A gas mixture consists of 4 kg of O2, 5 kg of N2, and 7 kg of CO2. Determine (a) the mass fraction o...
Questions in other subjects:


Mathematics, 04.04.2021 02:10

History, 04.04.2021 02:10

Arts, 04.04.2021 02:10


Mathematics, 04.04.2021 02:10




Mathematics, 04.04.2021 02:10