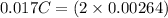Chemistry, 11.03.2020 22:15, hiiliohi1018
An aqueous solution of perchloric acid is standardized by titration with a 0.191 M solution of barium hydroxide. If 13.8 mL of base are required to neutralize 17.0 mL of the acid, what is the molarity of the perchloric acid solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
Do you know the correct answer?
An aqueous solution of perchloric acid is standardized by titration with a 0.191 M solution of bariu...
Questions in other subjects:











 solution is 0.311 M
solution is 0.311 M
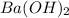 neutralizes 2 moles of
neutralizes 2 moles of 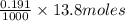 =
= 
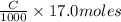 =
=