
Chemistry, 11.03.2020 13:33, lildanielmabien
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with excess HCl according to the following equation.
Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(l)
Group of answer choices
72.9 g
155 g
65.4 g
16.3 g
32.6 g

Answers: 1
Other questions on the subject: Chemistry


Do you know the correct answer?
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with excess HCl according to...
Questions in other subjects:


Geography, 08.02.2022 20:30


English, 08.02.2022 20:30


Chemistry, 08.02.2022 20:30

Social Studies, 08.02.2022 20:30


Mathematics, 08.02.2022 20:30


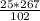 = 65.4 g of AlCl₃.
= 65.4 g of AlCl₃.




