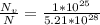Chemistry, 10.03.2020 07:42, gudon986732
For some hypothetical metal, the equilibrium number of vacancies at 600°C is 1 × 1025 m-3. If the density and atomic weight of this metal are 7.40 g/cm3 and 85.5 g/mol, respectively, calculate the fraction of vacancies for this metal at 600°C.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2



Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
Do you know the correct answer?
For some hypothetical metal, the equilibrium number of vacancies at 600°C is 1 × 1025 m-3. If the de...
Questions in other subjects:

History, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00

Computers and Technology, 24.06.2019 13:00

English, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00


Mathematics, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00



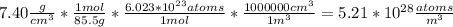
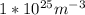 N is the number of atoms per volume calculated above.
N is the number of atoms per volume calculated above.