Chemistry, 04.03.2020 19:17, lberries08
Uppose you perform a titration of an unknown weak acid solution. You start with 4.00 mL of the weak acid and find that it takes 12.8 mL of 0.0500 M NaOH to reach the equivalence point. What is the concentration of the unknown weak acid solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 23.06.2019 15:00, WampWamp8751
For part 1, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least copper(ii) nitrate to the well with the most copper(ii) nitrate. which wells had the most distinct precipitate? for part 2, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least iron(ii) sulfate to the well with the most iron(ii) sulfate. which wells had the most distinct precipitate? for part 3, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least iron(iii) nitrate to the well with the most iron(iii) nitrate. which wells had the most distinct precipitate?
Answers: 3

Chemistry, 23.06.2019 16:30, alisharodriguez1
Ashell can hold a maximum of 32 electrons, what is the value of n?
Answers: 3
Do you know the correct answer?
Uppose you perform a titration of an unknown weak acid solution. You start with 4.00 mL of the weak...
Questions in other subjects:





Mathematics, 12.02.2021 14:00


Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00



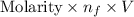
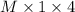 .......... (1)
.......... (1)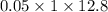 ............ (2)
............ (2)