Consider the reaction:
2al(s) + fe2o3(s) - al2o3(s) + 2fe(s)
the ah, for fe2o3(s) = -82...

Chemistry, 24.01.2020 23:31, SignoraPenguino
Consider the reaction:
2al(s) + fe2o3(s) - al2o3(s) + 2fe(s)
the ah, for fe2o3(s) = -824.3 kj/mole. the ah, for al2o3(s) = -1675.7 kj/mole.
finish the equation.
ahxn = [(1)
c
y
kj/mole) + (2)
y kj/mole)] - [(1)
kj/mole) + (2) (
kj/mole)]

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 23.06.2019 03:30, Ramann03
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 15:30, expeditionofsin
How many moles of potassium nitrate, kno3 are present in a sample with a mass of 85.2 g?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 01.02.2021 23:00



Physics, 01.02.2021 23:00

Biology, 01.02.2021 23:00


Chemistry, 01.02.2021 23:00


Mathematics, 01.02.2021 23:00

Mathematics, 01.02.2021 23:00



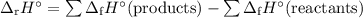
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ} & = & [1(-1675.7) + 2(0)] - [2(0) - 1(-824.3)]\\& = & -1675.7 + 824.3\\& = & \textbf{-851.4 kJ/mol}\\\end{array}\\\text{The enthalpy change is } \large \boxed{\textbf{-851.4 kJ/mol}}](/tpl/images/0469/5682/d5fae.png)




