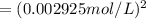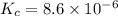Chemistry, 20.01.2020 21:31, amandanunnery33
Titanium(iv) chloride decomposes to form titanium and chlorine, like this: (l)(s)(g) at a certain temperature, a chemist finds that a reaction vessel containing a mixture of titanium(iv) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount calculate the value of the equilibrium constant for this reaction. round your answer to significant digits.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3

Do you know the correct answer?
Titanium(iv) chloride decomposes to form titanium and chlorine, like this: (l)(s)(g) at a certain t...
Questions in other subjects:

English, 11.05.2021 03:30







Mathematics, 11.05.2021 03:30



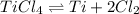
 4.18 g
4.18 g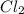 1.08g
1.08g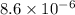 the value of the equilibrium constant for this reaction.
the value of the equilibrium constant for this reaction.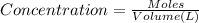
![[TiCl_4]=\frac{4.18 g}{190 g/mol\times 5.2 L}=0.004231 mol/L](/tpl/images/0463/1030/e0fe7.png)
![[Ti]=\frac{1.32 g}{48 g/mol\times 5.2 L}=0.005288 mol/L](/tpl/images/0463/1030/9e0bf.png)
![[Cl_2]=\frac{1.08 g}{71 g/mol\times 5.2 L}=0.002925 mol/L](/tpl/images/0463/1030/2b02a.png)
![K_c=[Cl_2]^2](/tpl/images/0463/1030/ae7ed.png)