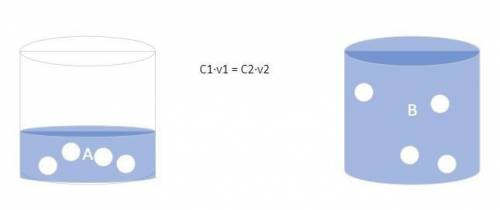
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 18.0 g of luminol to h2o creating a solution with a total volume of 75.0 ml. what is the molarity of the stock solution of luminol? before investigating the scene, the technician must dilute the luminol solution to a concentration of 5.00×10^-2 m. the diluted solution is then placed in a spray bottle for application on the desired surfaces. how many moles of luminol are present in 2.00 l of the diluted spray? what volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, britotellerialuis
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1


Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
Do you know the correct answer?
The forensic technician at a crime scene has just prepared a luminol stock solution by adding 18.0 g...
Questions in other subjects:

Spanish, 16.12.2021 17:30

Mathematics, 16.12.2021 17:30


Mathematics, 16.12.2021 17:30

Business, 16.12.2021 17:30

Biology, 16.12.2021 17:30