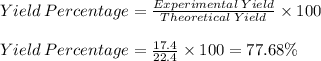Chemistry, 05.12.2019 08:31, LilFlamingo247
Consider the balanced chemical reaction below and determine the percent yield for iron if 11.2 moles of iron(iii) oxide yielded 17.4 moles of iron
fe2o3+3co> 2fe+3co2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
Do you know the correct answer?
Consider the balanced chemical reaction below and determine the percent yield for iron if 11.2 moles...
Questions in other subjects:




Mathematics, 24.06.2019 00:30




Mathematics, 24.06.2019 00:30




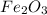 yields 2 moles of Fe.
yields 2 moles of Fe.