
Chemistry, 26.11.2019 04:31, emiliolovato
The maximum allowable concentration of pb2+ ions in drinking water is 0.05 ppm (i. e., 0.05 g of pb2+ in 1 million grams of water). (a) what is the pb2+ concentration of an underground water supply at equilibrium with the mineral cerussite (pbco3) (ksp = 3.3 × 10−14) ? × 10 g / lenter your answer in scientific notation. (b) does this concentration exceed the allowable concentration guideline? yes no

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, irene4523
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2
Do you know the correct answer?
The maximum allowable concentration of pb2+ ions in drinking water is 0.05 ppm (i. e., 0.05 g of pb2...
Questions in other subjects:





Chemistry, 19.03.2021 19:10


Chemistry, 19.03.2021 19:10



Mathematics, 19.03.2021 19:10


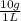 ×
×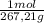 = 0,0374M
= 0,0374M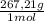 ×
×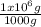 = 0,049 g of Pb²⁺ in 1 million grams of water
= 0,049 g of Pb²⁺ in 1 million grams of water



