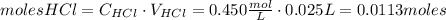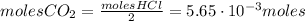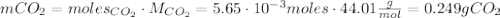Chemistry, 19.11.2019 06:31, caromaybelline71
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the following equation: caco3(s) 2hcl(aq)⟶co2(g) h2o(l) cacl2(aq) a typical antacid contains caco3. if such an antacid is added to 25.0 ml of a solution that is 0.450 m in hcl, how many grams of co2 gas are produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
Do you know the correct answer?
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the followi...
Questions in other subjects:






Biology, 23.06.2021 02:30