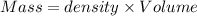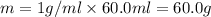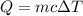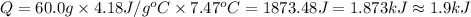Chemistry, 19.11.2019 02:31, Miloflippin9766
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.47 was observed. the specific heat of water is 4.18 j/(g * ∘c) calculate the heat of reaction (in kilojoules). record your answer with the proper significant figures and include the correct sign if needed. assume the density and specific heat of the solution are the same as that of water. kj

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, braydentillery1221
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1


Chemistry, 22.06.2019 08:00, katelyn0579
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely. analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
Do you know the correct answer?
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.4...
Questions in other subjects:


Mathematics, 07.08.2019 04:10

Mathematics, 07.08.2019 04:10


Mathematics, 07.08.2019 04:10

Mathematics, 07.08.2019 04:10