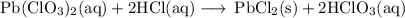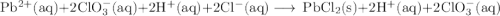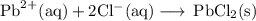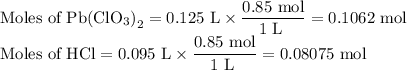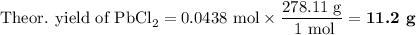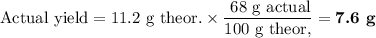Chemistry, 07.08.2019 04:10, kraigstlistt
Lead all chlorate is mixed with hydrolylic acid. each solution is 0.85 molar. write balanced, molecular, ionic, and net equations with state labels. use solubility rules and knowledge of strong acids. predict how many grams of what solid product can be collected if 125ml lead ll chlorate was treated with with 95 ml of the hydrologic acid. if percent yield was 68 percent how much product was collected and what is the molarity of the chlorate? what is the final concentration of pb2+. show work!

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1


Do you know the correct answer?
Lead all chlorate is mixed with hydrolylic acid. each solution is 0.85 molar. write balanced, molecu...
Questions in other subjects:


Mathematics, 28.08.2020 18:01

Mathematics, 28.08.2020 18:01



Computers and Technology, 28.08.2020 18:01

Mathematics, 28.08.2020 18:01

Biology, 28.08.2020 18:01


English, 28.08.2020 18:01