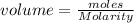a. moles = molarity × volume.

All of the statements about molarity are correct except :
a. moles = molarity × volume.
b. the molarity of a diluted solution is less than the molarity of the original solution.
c. the abbreviation is m. volume = moles/molarity.
d. the interpretation of the symbol is "moles of solute per mole of solvent."

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
Do you know the correct answer?
All of the statements about molarity are correct except :
a. moles = molarity × volume.
a. moles = molarity × volume.
Questions in other subjects:

Mathematics, 16.11.2020 23:10

Mathematics, 16.11.2020 23:10


Business, 16.11.2020 23:10




Mathematics, 16.11.2020 23:10

Mathematics, 16.11.2020 23:10

Mathematics, 16.11.2020 23:10


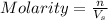
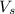 = volume of solution in L
= volume of solution in L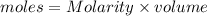
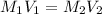
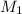 = molarity of stock solution
= molarity of stock solution 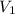 = volume of stock solution
= volume of stock solution 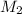 = molarity of diluted solution
= molarity of diluted solution 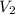 = volume of diluted solution
= volume of diluted solution