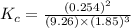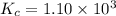Amixture of hydrogen and nitrogen, which produces ammonia (nh3) in a reaction vessel, is allowed to reach equilibrium at a given temperature. the equilibrium mixture of gases contained 0.254 m nh3, 1.85 m n2, and 9.26 m h2. calculate the equilibrium constant, kc at this temperature.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
Do you know the correct answer?
Amixture of hydrogen and nitrogen, which produces ammonia (nh3) in a reaction vessel, is allowed to...
Questions in other subjects:



History, 11.03.2020 05:10

Mathematics, 11.03.2020 05:10




Mathematics, 11.03.2020 05:11


Mathematics, 11.03.2020 05:11


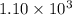
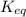
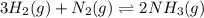
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0379/6771/c3aa0.png)